For decades, rheumatoid arthritis (RA) was seen as a slow, unstoppable force-destroying joints, crushing mobility, and wearing down lives. But today, something has changed. Biologic DMARDs have turned what was once a life sentence into a manageable condition, and for many, remission is no longer a dream-it’s a real possibility.
What Are Biologic DMARDs and How Do They Work?
Biologic DMARDs, or disease-modifying antirheumatic drugs, are not your grandfather’s arthritis pills. Unlike older drugs like methotrexate that broadly suppress the immune system, biologics are precision tools. They target specific parts of the immune system that go haywire in RA. Think of them as sniper rifles instead of scatterguns. These drugs were first approved in the late 1990s, starting with etanercept (Enbrel), which blocks tumor necrosis factor (TNF)-a key inflammation trigger. Since then, we’ve seen a whole family of biologics emerge. Some block TNF (adalimumab, infliximab, golimumab), others target interleukin-6 (tocilizumab), T-cells (abatacept), or B-cells (rituximab). Even newer options like tofacitinib and upadacitinib, though technically synthetic, work similarly by blocking signaling pathways inside immune cells. The goal isn’t just to ease pain. It’s to stop the immune system from attacking your joints. When that happens, joint damage slows-or even stops. And for a significant number of people, inflammation drops so low that symptoms vanish. That’s remission.Who Gets Biologic DMARDs and When?
Not everyone with RA starts with a biologic. The American College of Rheumatology recommends methotrexate as the first step for most patients. It’s cheap, well-studied, and works for many. But if after 3-6 months of methotrexate, you’re still in pain, swollen, or showing signs of joint damage on scans, it’s time to talk about biologics. About 30% of RA patients eventually need a biologic. That number rises if you’re diagnosed young, have high levels of inflammation markers like CRP or ESR, or already have joint erosion visible on X-rays. These are the people who benefit most from early biologic use. Doctors don’t just pick one at random. They look at your disease pattern, your other health conditions, and even your lifestyle. For example, if you’ve had tuberculosis or hepatitis B, some biologics are off-limits because they can reactivate old infections. If you’re planning to get pregnant, certain drugs like rituximab are safer than others.How Effective Are Biologic DMARDs?
The numbers speak for themselves. In clinical trials, 5-15% of people on methotrexate alone reach remission. With biologics, that jumps to 20-50%. Some studies show adalimumab and etanercept are slightly more effective than infliximab in real-world settings. And non-TNF biologics like abatacept and tocilizumab often outperform TNF blockers in patients who didn’t respond to the first round. One 2023 study from the Swiss RA registry found that baricitinib-a JAK inhibitor-achieved 28% higher remission rates than older biologics in patients who hadn’t responded to methotrexate. That’s not a small gain. It’s a game-changer for people who’ve tried everything else. But effectiveness isn’t the same for everyone. Biomarkers matter. If your joint tissue has low B-cell activity, rituximab might do almost nothing for you. But if your inflammation is driven by IL-6, tocilizumab could be a miracle. That’s why some clinics now test synovial tissue before choosing a biologic-though it’s still not routine everywhere.
What Are the Downsides?
Biologics aren’t magic. They come with real risks. The biggest concern is infection. Because these drugs turn down your immune system, you’re more vulnerable to pneumonia, skin infections, and even tuberculosis. That’s why everyone gets screened for TB before starting. You also need to avoid live vaccines while on biologics. Side effects vary. Injection site reactions are common with subcutaneous drugs like adalimumab-redness, itching, swelling. About 45% of patients report this. Infusion reactions (from IV drugs like infliximab) can cause chills, nausea, or headaches during treatment. Cost is another heavy burden. In the U.S., a year of biologic therapy can cost $50,000 to $70,000. That’s 5 to 10 times more than methotrexate. Insurance often requires step therapy-trying cheaper drugs first-and authorization can take 7 to 14 days. Many patients delay treatment because of paperwork, not because they’re not sick enough. And then there’s the problem of losing response. About 40% of patients who initially benefit from a biologic start to feel worse again after 12-24 months. This is called secondary non-response. When that happens, switching to another biologic helps-but each new switch tends to work less well than the last.Biosimilars: The Cost-Saving Game Changer
Here’s some good news: biosimilars are here. Biosimilars are near-identical copies of brand-name biologics, approved after the original patents expired. The first TNF biosimilars hit the U.S. market in 2016. Now, they make up 35% of TNF inhibitor prescriptions. And they cost 15-30% less. Patients on biosimilars report similar effectiveness and side effects as those on the originals. One Reddit user wrote: “Switched from Humira to its biosimilar. Same results, $1,200 less per month out of pocket.” Still, some patients worry about switching. Will it work the same? Will their body react differently? Studies show no major difference in safety or response. The FDA and EMA both approve biosimilars only after rigorous testing. If your doctor suggests switching, it’s not a downgrade-it’s a smarter choice.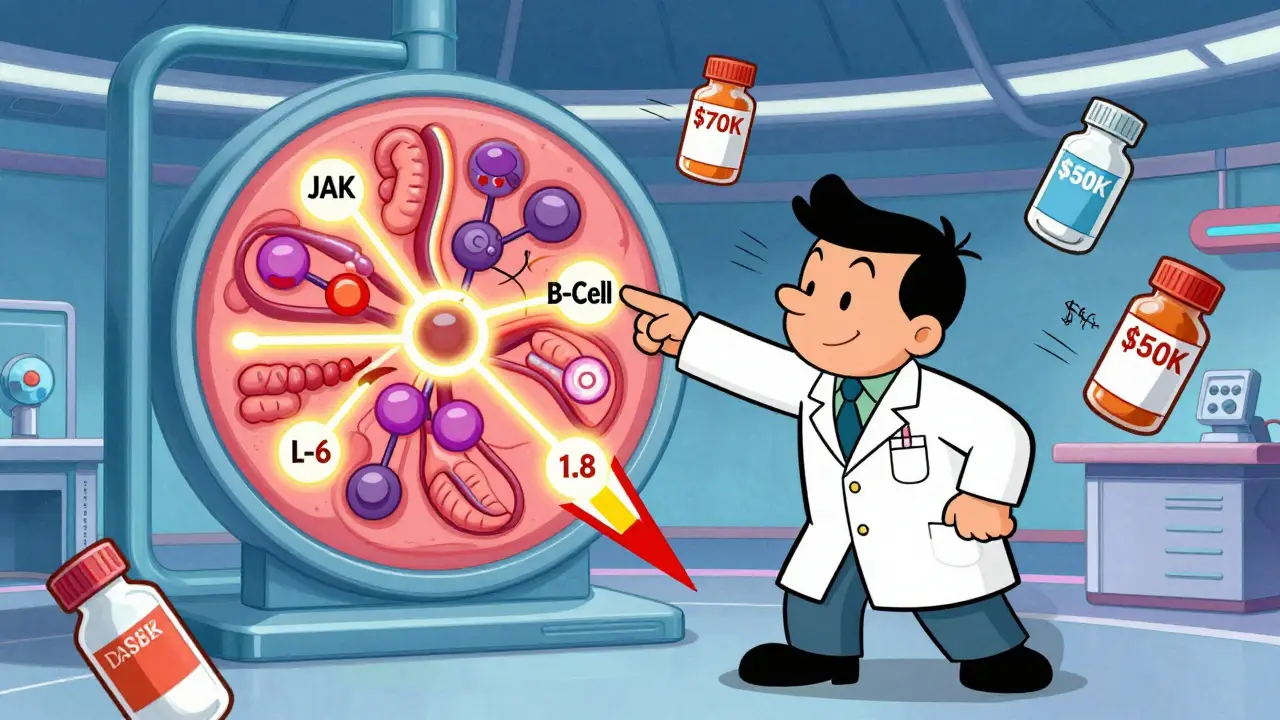
Real Stories: Remission Is Possible
On patient forums, stories of remission are everywhere. One woman, diagnosed with severe RA at 28, spent 15 years in pain. She tried six different drugs. Nothing worked. Then she started tocilizumab. Within eight weeks, her joints stopped swelling. Her ESR dropped from 87 to 8. She’s been in remission for five years. Another man, a mechanic, couldn’t grip tools. After starting adalimumab, he returned to work full-time. He still gets injections every two weeks, but now he plays with his grandkids without pain. These aren’t outliers. They’re the new normal.How to Stay on Track
Getting a biologic is just the start. Staying on it is what matters. Most biologics are injected at home-weekly or every other week. The first time can be scary. But 75% of patients learn to self-administer after just two training sessions with a nurse. Many manufacturers offer free coaching, injection kits, and even apps that remind you when to take your dose. You also need to track your disease. Doctors use tools like DAS28-a score based on swollen joints, pain levels, and blood tests-to measure progress. Remission means a DAS28 score below 2.6. If your score creeps up, don’t wait. Call your rheumatologist. Don’t skip appointments. Blood tests for liver function, blood counts, and infections are required every few months. Skipping them can lead to serious problems. And if you’re struggling with cost? Ask about patient assistance programs. Most drugmakers offer discounts or even free medication for qualifying patients. Specialty pharmacies can help navigate insurance.The Future: Personalized RA Treatment
The next big leap isn’t just better drugs-it’s smarter choices. Researchers are now using blood and joint tissue tests to predict which biologic will work best for you. One study found that patients with high levels of certain proteins responded best to abatacept. Others with different markers did better with tocilizumab. Longer-acting versions are coming too. A twice-yearly injection of tocilizumab is in late-stage trials. Imagine fewer shots, less hassle, and just as much control. By 2027, biosimilars are expected to make up 60% of the biologic market. That means more access, lower costs, and more people reaching remission. The dream of curing RA might still be far off. But the goal of living without pain, without damage, without fear-that’s already here.Can biologic DMARDs really lead to remission in rheumatoid arthritis?
Yes. Studies show that 20-50% of RA patients achieve remission with biologic DMARDs, compared to only 5-15% with older drugs like methotrexate alone. Remission means little to no joint pain, swelling, or damage, and normal blood markers of inflammation. It’s not a cure, but it allows many people to live without daily symptoms.
How long does it take for biologic DMARDs to work?
TNF inhibitors like adalimumab and etanercept often start working in days to weeks-many feel less pain within 2-4 weeks. Non-TNF biologics like abatacept or rituximab may take 3-6 months to show full effects. Patience is key, but if you don’t see improvement after 3 months, talk to your doctor about switching.
Are biosimilars as effective as brand-name biologics?
Yes. Biosimilars are approved by the FDA and EMA only after proving they work just like the original biologic in clinical trials. Patients switching from Humira to its biosimilar report the same level of symptom control and side effects. The main difference is cost-biosimilars are typically 15-30% cheaper.
What are the biggest risks of using biologic DMARDs?
The biggest risk is serious infection, including tuberculosis, pneumonia, and skin infections. Before starting, you’ll be tested for latent TB and hepatitis. Other risks include injection site reactions (redness, itching), increased risk of certain cancers (rare), and, rarely, nervous system disorders. Not everyone has these side effects, but regular monitoring is essential.
Why do some people stop responding to biologics over time?
This is called secondary non-response. The body may develop antibodies against the drug, making it less effective. Or the disease may evolve, using different inflammatory pathways. About 40% of patients experience this after 12-24 months. The solution is switching to a biologic with a different mechanism-like moving from a TNF blocker to an IL-6 or JAK inhibitor.
Is it safe to stop biologic DMARDs once in remission?
Most doctors advise against stopping, even if you’re in remission. Stopping often leads to flare-ups within months. Some patients under close supervision may try to taper slowly, but this is only done with careful monitoring and rarely leads to lasting remission without medication. Think of biologics like insulin for diabetes-they manage the disease, but don’t fix the underlying cause.
How do I know if my biologic is working?
Track your symptoms: fewer swollen joints, less morning stiffness, lower pain levels. Your doctor will also use blood tests (CRP, ESR) and a scoring system called DAS28. A DAS28 score below 2.6 means remission. If your score stays high after 3 months, your treatment may need adjustment.
Can I take biologic DMARDs with methotrexate?
Yes, and often it’s recommended. Combining methotrexate with a biologic improves effectiveness and reduces the chance your body will develop antibodies against the biologic. Many patients stay on low-dose methotrexate even after starting a biologic. It’s not required for all, but it boosts results for most.

 Roccat Burst Pro Air Review: Revolutionizing Gaming with Lightweight, Wireless Design
Roccat Burst Pro Air Review: Revolutionizing Gaming with Lightweight, Wireless Design
 Esophageal Cancer Risk from Chronic GERD: Key Red Flags You Can't Ignore
Esophageal Cancer Risk from Chronic GERD: Key Red Flags You Can't Ignore
 Buy Generic Prilosec (Omeprazole) Online in Australia: Cheap, Safe Options for 2025
Buy Generic Prilosec (Omeprazole) Online in Australia: Cheap, Safe Options for 2025
 Floaters After Cataract Surgery: What’s Normal and What’s Not
Floaters After Cataract Surgery: What’s Normal and What’s Not
 Corticosteroid-Induced Hyperglycemia and Diabetes: How to Monitor and Manage It
Corticosteroid-Induced Hyperglycemia and Diabetes: How to Monitor and Manage It
Eileen Reilly
January 13, 2026 AT 10:35okay so i just switched to a biosimilar last month and holy shit my out-of-pocket dropped from $1400 to $200. no joke. i was scared as hell but my joints feel the same, no weird side effects. if your doc pushes it, just say yes. no need to be a hero with brand names.
Monica Puglia
January 14, 2026 AT 03:43my mom’s been in remission for 4 years on tocilizumab 🥹 she went from needing help to tie her shoes to hiking with the grandkids. it’s not a cure but it’s the closest thing we’ve got. if you’re scared to start, just remember: your body isn’t the enemy, your immune system is. this helps fix that.
steve ker
January 15, 2026 AT 18:13Rebekah Cobbson
January 17, 2026 AT 04:06if you’re reading this and you’re nervous about starting a biologic - you’re not alone. i cried before my first injection. but the nurse walked me through it, i did it at home while watching netflix, and now i’m gardening again. it’s not magic but it’s real. you deserve to move without pain.
Lauren Warner
January 17, 2026 AT 16:46let’s be real - the pharmaceutical industry is milking this. yes biologics work but they’re overpriced, overhyped, and the side effects are downplayed. i’ve seen people get fungal infections, lymphoma, and then get blamed for ‘not following protocol’. it’s not a miracle - it’s a corporate profit machine wrapped in a white coat.
and don’t get me started on biosimilars. same drug, same risks, but now they’re pushing you to switch so they can cut costs. you’re not a number. your health isn’t a spreadsheet.
Craig Wright
January 18, 2026 AT 15:10It is frankly unacceptable that the United States continues to allow such exorbitant pricing for life-altering medications. In the United Kingdom, we have long since implemented cost-controlled access through the NHS. The fact that patients must endure bureaucratic delays for essential treatment is a moral failure of the American healthcare system.
Furthermore, the reliance on biologics reflects a broader trend of medical overcomplication. Simplicity, discipline, and physical therapy should be prioritized before resorting to immunosuppressive agents.
Rinky Tandon
January 20, 2026 AT 07:48let me drop some truth bombs. if you're on a TNF blocker and not in remission by month 3 - you're wasting time. the cytokine profiling isn't optional anymore. you need IL-6 vs JAK vs B-cell signature analysis - otherwise you're just shooting in the dark. and if your rheum is still using DAS28 like it's 2010? fire them. synovial biopsy is the new gold standard. no more guesswork. precision medicine isn't a buzzword - it's survival.
also - methotrexate combo? non-negotiable. monotherapy is for people who don't want remission. and biosimilars? yes they're equal. the data is ironclad. stop being a Luddite.
Ben Kono
January 21, 2026 AT 05:31Cassie Widders
January 22, 2026 AT 07:28i’ve been on adalimumab for six years. still in remission. still get my shots. still play with my dog. it’s not glamorous but it’s my normal now. if you’re scared - start slow. talk to someone who’s been there. you’ve got this.